AMDI Q-NAb™ IgG Kit Fast Quantitative Detection of Neutralizing Activity Against SARS-CoV-2 For Research Use Only
Why not use Live Virus Assays?
Although considered the gold standard, live virus neutralization assays are rarely used outside of virology research and vaccine development due to their associated time and cost:- Long turnaround times (weeks or months)
- High cost (~$750-$1000/sample)
- Requires a BSL-3 facility
- Specially trained laboratory technicians needed
AMDI Q-NAb IgG Kit Dynamic Range
- WHO Traceable
- Quantitation of Spike and Nucleocapsid proteins (NP) is not affected by the Fusion Protein allowing for multiplexing capability
- Wide Dynamic Range
- BA4/5 NAb titer: 300-30,000 IU/mL
- Wuhan titer: 70-7500 IU/mL
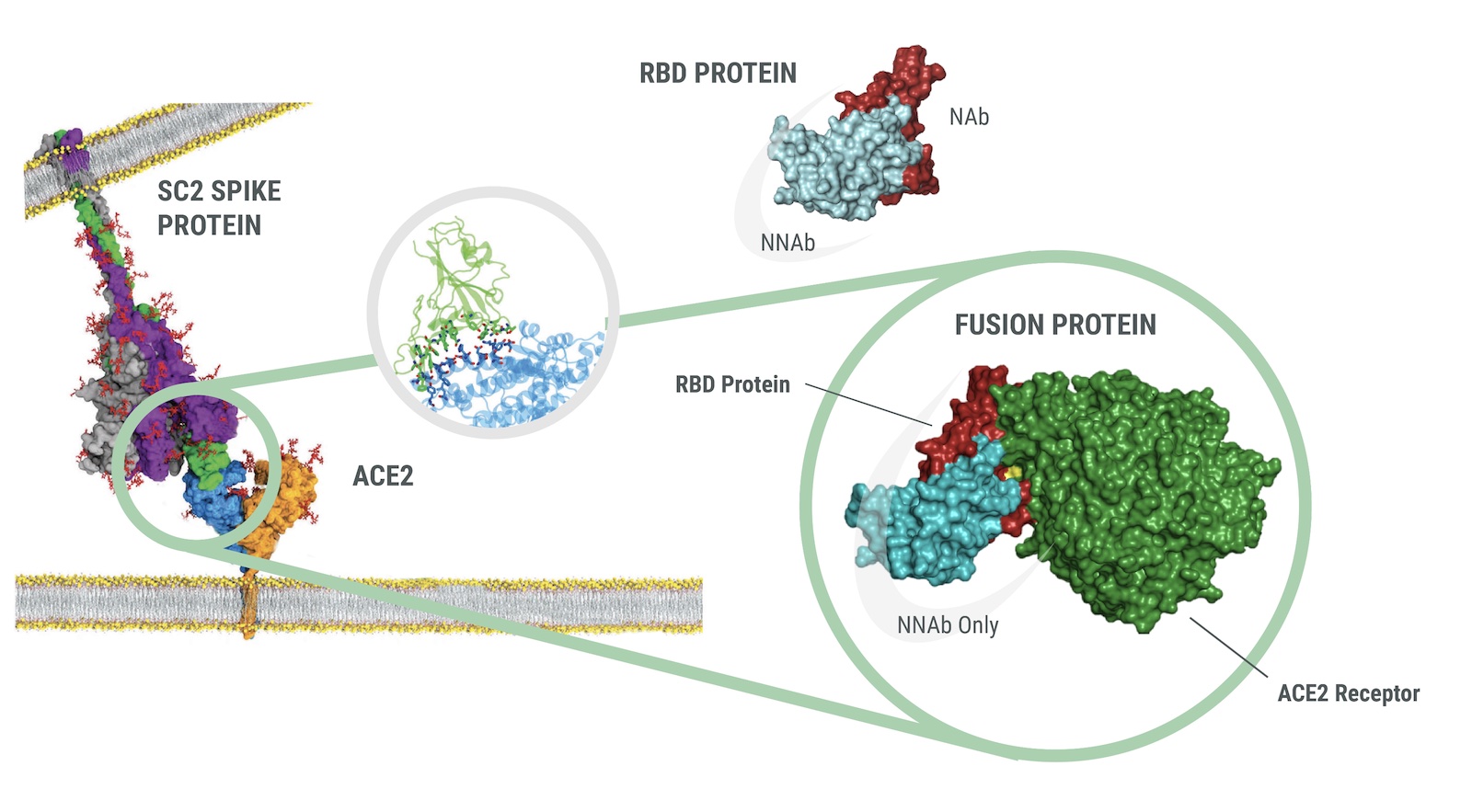
The Q-NAb IgG Kit uses Fusion Protein (FP) that mimics Spike receptor binding domain (RBD) docked with Human ACE2 to bind Non-Neutralizing Antibodies (NNAbs). The docked format of the FP is held in the closed configuration such that the receptor binding motif (RBM) is hidden due to the interaction of the RBD with human ACE2 while the rest of the RBD is exposed. When incubated with a clinical sample, the FP acts as an effective blocker of NNAbs.
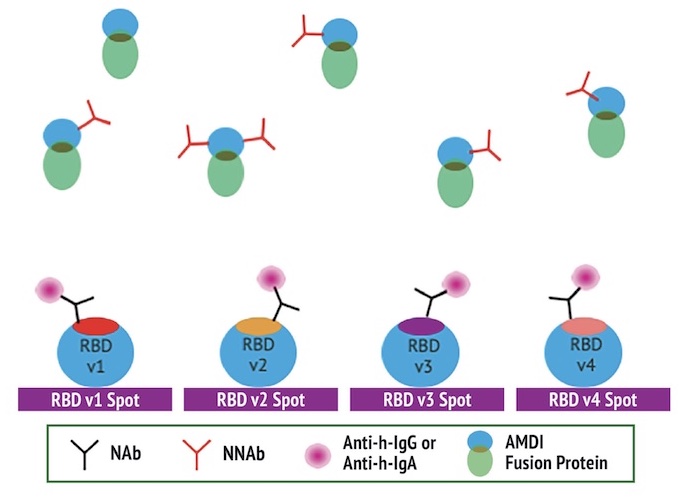
The Q-NAb IgG Test Principle
The quantitative Q-NAb lgG assay was designed to directly detect NAbs binding to SARS-CoV-2 RBD variants on plates by first sequestering NNAbs using FP. The use of a universal FP and engineered RBDs allows the detection of NAbs against multiple RBD variants simultaneously. The Q-NAb lgG plate assay was developed to detect NAb’s against ancestral SARS-CoV-2 and the BA.4/5 variant.